
 The Language of Chemistry The Language of Chemistry |
|||||
| Atoms, Molecules & Ions | Chemical Formulae & Valency |
The Language of Chemistry - 01
Atoms, Molecules & Ions
Atoms
As we have seen in Introduction to the Periodic Table , the smallest particle of an element that maintains its chemical identity through all chemical and physical changes is an atom. Atoms are made up of three fundamental particles: the electron, the proton, and the neutron. The nucleus at the centre of the atom consists of protons and neutrons, while the electrons zip around the nucleus. An electron carries a negative charge (denoted as e−), a proton carries a positive charge (denoted as p+), while the neutron is electrically neutral.
Since an atom is electrically neutral, it contains an equal numbers of electrons and protons, such that the negative and positive charges balance each other out.
An element's unique properties is determined by the number of protons in its atom. The number of protons in an atom of an element is known as its atomic number (Z). All atoms of an element contains the same number of protons. By extension, since the atoms are neutral, they also contain the same number of electrons.
Thus, for a neutral atom, the atomic number is given by
An atom’s mass number (A) is defined as the sum of the number of protons (Z) and the number of neutrons (N) in the atom.
Isotopes
Elements with same atomic numbers but different mass numbers are called isotopes. This implies that though the number of protons (and hence electrons) are the same, the number of neutrons are different.
For example, hydrogen has three isotopes:
- protium ( ) or normal hydrogen: Its nucleus consists of just 1 proton, and no neutrons.
- deuterium (): Its nucleus has 1 proton and 1 neutron.
- tritium ( ) : It has 1 proton and 2 neutrons.
| Element | Symbol |
|---|---|
| aluminium | Al |
| argon | Ar |
| barium | Ba |
| boron | B |
| bromine | Br |
| calcium | Ca |
| carbon | C |
| chlorine | Cl |
| fluorine | F |
| helium | He |
| hydrogen | H |
| iodine | I |
| lithium | Li |
| magnesium | Mg |
| manganese | Mn |
| nitrogen | N |
| oxygen | O |
| phosphorus | P |
| silicon | Si |
| sulphur | S |
| zinc | Zn |
| copper (cuprum) | Cu |
| iron (ferrum) | Fe |
| lead (plumbum) | Pb |
| mercury (hydrargyrum) | Hg |
| potassium (kalium) | K |
| silver (argentum) | Ag |
| sodium (natrium) | Na |
However, you should know that the chemical properties of an element is totally dependent on the number of electrons that its atom contains, which itself depends on the number of protons the atom has. The number of neutrons do not affect its chemical properties. All the three isotopes of hydrogen exhibit identical chemical reactions.
Carbon also has 3 isotopes –
- carbon-12 () or normal carbon,
- carbon-13 (), and
- carbon-14 ().
Chemical Symbol and Nuclide Symbol
The chemical symbol of an element represents 1 atom of the element. A nuclide symbol consists of an element's symbol with atomic number and mass number indicated. For normal carbon or carbon-12, the symbol is C, while the nuclide symbol is as shown below:
Names and symbols of some common elements are shown in Table .
Molecules and Ions
It is the electrons which form the connections, or chemical bonds, that join atoms together to form chemical compounds. Chemical bonds between atoms are usually classified as either covalent bonds or ionic bonds.
Covalent Bonds & Molecules
A covalent bond is the most common kind of chemical bond. It results from atoms sharing some electrons. The result of a covalent bond between two or more atoms is a molecule. As a general rule, covalent bonds occur between non-metal atoms.
Consider a few examples:
- A molecule of hydrogen chloride, HCl, forms when 1 hydrogen atom and 1 chlorine atom share electrons.
- A water (hydrogen monoxide) molecule, H2O, results when 2 hydrogen atoms share electrons with 1 oxygen atom.
- An ammonia molecule, NH3, results from 3 hydrogen atoms sharing electrons with 1 nitrogen atom.

3-D models of molecules help in visualisation of the molecular structures. Fig. shows two kinds of models – the ball-and-stick models which emphasise the covalent bonds, and the space-filling models which accurately depict the overall molecular shape.
Atomicity
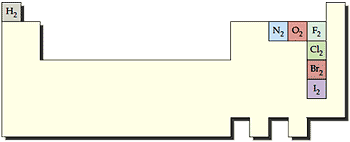
In normal state, some elements exist as molecules by forming covalent bonds, rather than as separate atoms. Hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine and iodine all exist as diatomic (two atom) covalently bonded molecules (Fig. ). Hence, symbolically, they are depicted as H2, N2, O2, F2, Cl2, Br2 and I2. Atomicity of an element is defined as the number of atoms in one molecule of the element.
Ionic Bonds & Formula Units
Unlike a covalent bond, an ionic bond results from transfer of one or more electrons from one atom to another. As a general rule, ionic bonds forms between atoms of metals (which tend to give up electrons) and non-metals (which tend to accept electrons).
An ion is an atom or group of atoms that carries an electric charge. Ions that are positively charged are called cations, while those that are negatively charged are called anions. The ionic bonds result from strong electrostatic forces of attraction between anions and cations.
Let us consider a couple of examples to illustrate the formation of ionic bonds.
Ionic Solids
An important thing to note about ionic bonding is that the formation of ionic units like Na+Cl− does not mean that NaCl is a discrete "molecule." What actually happens is the formation of an ionic solid. Here's how.

As is obvious, there will be billions of Na+ cations and Cl− anions. Opposite charges attract, so each Na+ will try to attract as many Cl− around itself as it can, while repelling other Na+ cations. Meanwhile, each Cl− will be trying to attract Na+ cations and repelling other Cl− anions. The final result is an ionic solid, where the ions arranged in a regular, cubic arrangement (in the case of sodium chloride) in which each Na+ has six Cl− as its nearest neighbours, and each Cl− has six Na+ as its nearest neighbours. The structure is as depicted in Fig. .
Formula Units
As you have seen, there is nothing like discrete "molecules" in the case of ionic solids. However, it is helpful in chemistry to consider the fundamental unit formed out of ionic bonding (such as NaCl) as a "molecule," only that it is called a formula unit. In fact, the term formula unit is a more generic term and can also be used to refer to a molecule of a substance with covalent bonds
Polyatomic Ions or Radicals
Polyatomic ions, or radicals, are covalently bonded groups of atoms which are charged and work as a single unit in chemical reactions. Some examples of radicals are:
- ammonium ion (NH4+),
- hydroxide ion (OH−), also called hydroxyl ion,
- nitrate ion (NO3−), and
- sulphate ion (SO42−)
- sulphate ion (SO42−)
As you will be learning later, when writing the formulas of substances containing more than one of these radicals, parentheses are placed around the entire polyatomic unit.
List of References
Bibliography
| Atoms, Molecules & Ions | Chemical Formulae & Valency | ||||
 The Language of Chemistry The Language of Chemistry |
 High School
High School Mathematics
Mathematics Sets & Basic Operations
Sets & Basic Operations