
 Matter Matter |
|||||
| Laws of Chemical Combination & Dalton's Atomic Theory | Kinetic Theory of Matter | Properties of Gases & Gas Laws |
Matter - 04
Kinetic Theory of Matter
The kinetic theory of matter attempts to explain the physical properties of matter (in its various states) in terms of the motion of its particles.
The main aspects of the kinetic theory are:
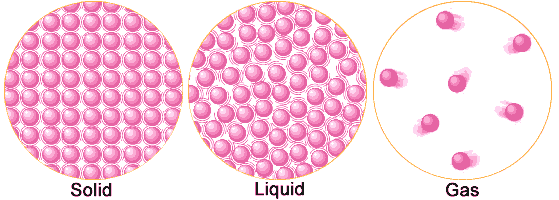
- Matter is composed of very tiny particles (atoms or molecules), which are separated from each other by interparticle distances. (see Fig. )
- Each particle of matter is in constant motion.
- In a gas, the particles can move around freely and independently.
- In a liquid, particle movement is a bit constrained and limited to sliding/flow movement within its volume.
- In a solid, particle movement is fully constrained and restricted to only vibrational motion of particles in their fixed positions within the solid.
- The particles of matter experience forces of attraction amongst themselves. These attractive forces decrease rapidly with increasing distance between the particles.
- Particles in solids are very close to each other, and the attractive forces are large enough to hold the particles in fixed positions. Thus, a solid has a fixed shape and a fixed size (volume).
- The particles of liquids are a little further apart and are free to slide and flow, taking the shape of the container. Thus, a liquid has no fixed shape. However, since the particle movement is restricted to within the space occupied by the liquid, a liquid does have a fixed size (volume).
- The separations between particles of a gas are quite large, resulting in complete freedom of motion. Hence, a gas has neither fixed shape nor fixed size (volume), and tends to expand to fill up the entire volume of its container.
- Because the particles are in motion, they possess kinetic energy. The temperature of matter is a measure of the average kinetic energy possessed by the particles. When heat is applied to matter, it gets absorbed and translated to increased kinetic energy of the particles (which means greater motion), resulting in a rise in temperature.
Change of State and Kinetic Theory
The kinetic theory of matter gives a clear explanation of the internal processes involved at the particle level when matter undergoes a change of state.
Process of heating
Theoretically, heating a solid to higher and higher temperatures changes its phase to a liquid, and finally to a gas.
Fusion (solid to liquid)
A solid consists of low kinetic energy vibrating particles locked into position by interparticle attractive forces. When heat is applied, energy is absorbed and the particles start vibrating more vigorously.
Finally, the vibrations become energetic enough to overcome the attractive forces, and the particles start sliding out of their positions to flow about. The solid is now melting into a liquid.
Vaporisation (liquid to gas)
On further application of heat to the liquid, the particles move around more energetically within the volume of the liquid. Finally, they become energetic enough to start escaping from the surface of the liquid, overcoming the backward pull by their neighbours in the volume of the liquid.
The process of boiling has begun, wherein the liquid converts to gas as particles escape to move around independently without any constraints.
Evaporation (liquid to gas)
According to the kinetic theory, the temperature is a measure of the average kinetic energy possessed by the particles of matter. This means that in any sample of matter, there will be particles with higher kinetic energy than average, balanced by those with lower energy than average.
So, even in a liquid whose temperature is not high enough for boiling to occur, there will be some particles with sufficient kinetic energy to break through the surface of the liquid overcoming the backward pull of others. They slowly escape as gas particles, and the process is called evaporation.
Process of cooling
Generally, cooling a gas changes its phase to a liquid, and finally to a solid.
Condensation (gas to liquid)
When a gas is cooled (i.e. heat is removed) progressively, the free moving particles start losing kinetic energy and slowing down.
Finally, the forces of attraction between the lower energy particles colliding with each other are strong enough to hold them together, and the gas begins to condense into liquid.
Solidification (liquid to solid)
The particles still have energy enough to slide about within the volume of the liquid, but further cooling lowers this energy further.
Finally, the mutual attractive forces overcome the low kinetic energies of the particles and lock them into fixed positions, where they continue to vibrate as the liquid freezes to solid.
List of References
Bibliography
| Laws of Chemical Combination & Dalton's Atomic Theory | Kinetic Theory of Matter | Properties of Gases & Gas Laws | |||
 Matter Matter |
 High School
High School Mathematics
Mathematics Sets & Basic Operations
Sets & Basic Operations