
 Matter Matter |
|||||
| Properties of Gases & Gas Laws | Introduction to the Periodic Table |
Matter - 06
Introduction to the Periodic Table
The Concept of Atoms
The smallest particle of an element that maintains its chemical identity through all chemical and physical changes is called an atom. Atoms are made up of three fundamental particles: the electron, the proton, and the neutron. These are the basic building blocks of atoms, and thus of all matter.
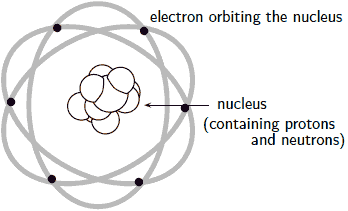
An electron carries a negative charge, a proton carries a positive charge, while the neutron is electrically neutral. Protons and neutrons are located at the centre of an atom bundled into an entity called the nucleus, while electrons zip around the nucleus in "orbits." These "orbits" are different from the elliptical/circular orbits of planets around the sun – how they are different will be covered later in the subject.
Fig. depicts a simple model of the atom, which was put forward by Rutherford. Although this model was later superseded by a better newer model, it nevertheless serves to clarify the basic structure of an atom.
Neutral atoms contain equal numbers of electrons and protons, such that the negative and positive charges balance each other out.
The difference between elements is determined by the number of protons in their atoms. The number of protons in an atom of an element is the element’s atomic number.
The atomic weight (nowadays referred to as atomic mass) of an element is the mass of one atom of that element with reference to the mass of a standard atom. Earlier, the mass of one atom of hydrogen was the standard against which atomic weights were measured.
Note that atomic weight (or atomic mass) is just a number.
Periodicity & the Periodic Law
In 1869, the Russian chemist Dmitri Mendeleev and the German chemist Lothar Meyer independently published arrangements of known elements that resemble the Modern Periodic Table in use today. While Mendeleev’s classification was based mainly on chemical properties of elements, Meyer based his largely on the physical properties. Both laid stress on periodicity (regular periodic repetition) of properties with increasing atomic weight.
Mendeleev arranged elements in rows and columns. Successive rows contained elements in increasing atomic weights, arranged such that elements having similar chemical properties fell in the same columns vertically through different rows. He also noted that the physical properties of elements too appeared to vary in a periodic fashion.
One of the great features of Mendeleev’s periodic table was that it contained empty slots for yet undiscovered elements. However, since in reality the properties of the elements vary with atomic number (the concept of atomic number was discovered 50 years after Mendeleev's work), it was found that there were several elements which did not fit into his periodic table since he had based the periodicity on atomic weights. Later, when it was finally discovered that it is the atomic number which is fundamental to an element's identity, and the elements were arranged according to their atomic numbers, the true form of periodicity emerged.
The periodic law tells us that if we arrange the elements in order of increasing atomic number we periodically find elements that have similar chemical and physical properties.
The Periodic Table
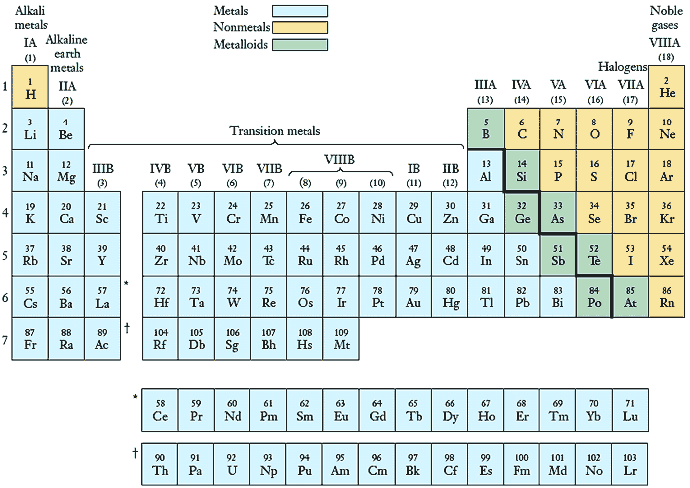
The presently used "long form" of the periodic table (Fig. ) is a tabular display of elements based on the periodic law. Each element is depicted by its symbol, with the number above the symbol representing the atomic number of the element.
In the periodic table, elements are arranged left to right and top to bottom in order of increasing atomic number, in such a way that the columns hold elements having similar physical and chemical properties. The vertical columns are referred to as groups or families, and the horizontal rows are called periods. Thus, elements in a group have similar chemical and physical properties, and those within a period have properties that change progressively across the table.
The older system of numbering of the groups (columns) is, in sequence: IA, IIA, IIIB, IVB, VB, VIB VIIB, VIIIB (which has 3 columns), IB, IIB, IIIA, IVA, VA, VIA, VIIA, VIIIA. Alternatively, the roman numerals are sometimes replaced by Arabic numerals (1A, 2A, 3B, 4B, 5B, 6B 7B, 8B, 1B, 2B, 3A, 4A, 5A, 6A, 7A, 8A). The newer system of numbering numbers the groups from 1 to 16. (Fig. )
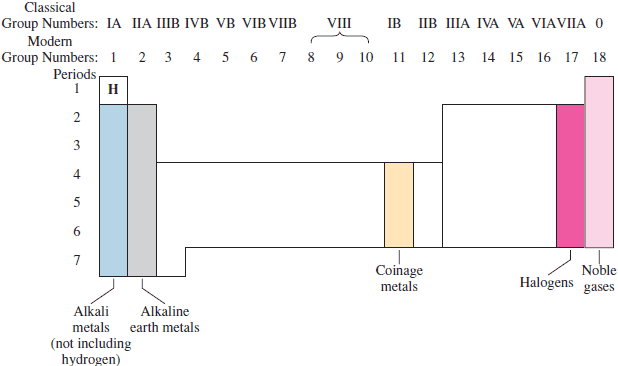
There are some important families of elements which you should know about (see Fig. ). Group IA elements, except for hydrogen (H), are known to as alkali metals, and the Group IIA elements are called the alkaline earth metals. The Group IB elements are referred to as the coinage metals, as they have been used for making coins. The Group VIIA elements are known as halogens, which means "salt formers," and the Group VIIIA elements are the so called noble gases (or rare gases). These will be covered in a little more detail further on.
Metals, Non-metals and Metalloids
Within a single group, the similarities between the elements are pronounced. However, neighbouring groups of elements also show some types of similarities. Based on this, the periodic table is often divided into three major classes of elements: metals, non-metals, and metalloids.
| Metals | Non-metals | |
|---|---|---|
| 1 | High electrical conductivity that decreases with increasing temperature | Poor electrical conductivity (except carbon in the form of graphite) |
| 2 | High thermal conductivity | Good heat insulators (except carbon in the form of diamond) |
| 3 | Metallic gray or silver luster 1 | No metallic luster |
| 4 | Almost all are solids 2 | Solids, liquids, or gases |
| 5 | Malleable | Brittle in solid state |
| 6 | Ductile | Non-ductile |
| 1 Except copper and gold 2 Except mercury; cesium and gallium melt in a protected hand |
||
Physical properties that distinguish metals from non-metals are summarised in . Metalloids are elements which behave both like metals and non-metals.
The classification of elements into these three classes is depicted in the periodic table (Fig. ). The elements to the left of those touching the heavy stair-step line are metals (except hydrogen), and those to the right are non-metals. Most elements adjacent to the heavy line are the metalloids.
Metals
Metals form the largest group of elements. All, except mercury, are solid at room temperature, and most have a silvery shine. In addition, metals are generally malleable and ductile, and are good conductors of heat and electricity.
Aluminum is the least metallic of the metals and is sometimes classified as a metalloid. It is metallic in appearance, and an excellent conductor of electricity.
Non-metals
There are seventeen non-metals, and at room temperature, eleven of them are gases, one is a liquid (bromine), and only five are solids (carbon, phosphorus, sulphur, selenium, and iodine). None are silvery in appearance, and most are brightly colored. The solid non-metals are brittle rather than malleable or ductile, and are poor conductors of heat and electricity.
Metalloids
Seven of the nine elements adjacent to the zigzag boundary (Fig. ) between metals and nonmetals—boron, silicon, germanium, arsenic, antimony, tellurium, and astatine—are known as metalloids or semi metals, because their properties are intermediate between those of their metallic and nonmetallic neighbors. Most are silvery in appearance. All are solid at room temperature. However, metalloids are brittle.
Many of the metalloids, such as silicon, germanium, and antimony, act as semiconductors (functioning as insulators at lower temperatures and as conductors at higher temperatures) and so are used in electronic circuits. The conductivities of metals, by contrast, decrease with increasing temperature.
Introduction to some periodic table families
Group IA – Alkali Metals
Hydrogen is a unique element. Although listed under Group IA, hydrogen is not a metal under ordinary conditions. Also, hydrogen sometimes behaves as though it belongs in Group VIIA.
The main alkali metals are: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), and cesium (Cs). They are shiny, soft metals. All react rapidly (often violently) with water to form products that are highly alkaline, or basic—hence the name alkali metals. The alkali metals, because of their high reactivity, are not found in nature in the pure state, but only in combination with other elements. The sixth alkali metal, francium (Fr), hardly exists in nature.
Group IIA – Alkaline Earth Metals
The alkaline earth metals are: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They are also shiny metals, but harder, and less reactive than their neighbors in Group IA. They too are never found in nature in the pure state.
Group IB – Coinage Metals
The coinage metals are: copper (Cu), silver (Ag) and gold (Au). Historically, they have been used for minting coins for use in economic transactions. They are malleable and ductile, and thus easy to mint. They are resistant to corrosion, which is another advantage.
Group VIIA – Halogens
The main halogens are: fluorine (F), chlorine (Cl), bromine (Br) and iodine (I), of which fluorine and chlorine are gases, bromine is a liquid and iodine is solid. They are colorful, corrosive nonmetals. They are found in nature only in combination with other elements, such as with sodium in sodium chloride (common salt). The fifth halogen, astatine (At) is almost non-existent in nature.
Group VIIIA – Noble Gases
The noble gases are: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). These gases hardly react with other elements, hence the name noble gases. Whereas helium, neon, and argon don’t combine with any other element, krypton and xenon combine with very few.
List of References
Bibliography
| Properties of Gases & Gas Laws | Introduction to the Periodic Table | ||||
 Matter Matter |
 High School
High School Mathematics
Mathematics Sets & Basic Operations
Sets & Basic Operations